Background: Glofitamab is a CD20xCD3 bispecific antibody with a 2:1 (CD20:CD3) format that engages and redirects T cells to eliminate B cells. In a pivotal Phase II study (NCT03075696), fixed-duration glofitamab monotherapy induced high complete response (CR) rates and had a manageable safety profile in patients with relapsed and/or refractory (R/R) large B-cell lymphoma (LBCL; Dickinson et al. N Engl J Med 2022). Here, we present an extended follow up, as well as subgroup analyses in patients with prior chimeric antigen receptor (CAR) T-cell therapy and by baseline total metabolic tumor volume (TMTV).
Methods: Patients with LBCL and ≥2 prior therapies received obinutuzumab pretreatment (1000mg) on Day (D) 1 of Cycle (C) 1. Intravenous glofitamab was then given as step-up doses during C1 (2.5mg on D8; 10mg on D15), followed by the target dose (30mg) on D1 of C2-12 (21-day cycles; total of 8.3 months). The primary endpoint was independent review committee (IRC)-assessed CR rate using Lugano criteria (Cheson et al. J Clin Oncol 2014). Cytokine release syndrome (CRS) events were assessed using American Society for Transplantation and Cellular Therapy criteria (Lee et al. Biol Blood Marrow Transplant 2019). Exploratory analyses were performed to investigate the association between TMTV at baseline and progression-free survival (PFS) and CRS. As there is currently no consensus on the method for standardized uptake value (SUV) thresholding in non-Hodgkin's lymphoma, a variety of methods are used in clinical studies (Keijzer et al. Comput Struct Biotechnol J 2023). Here, we used IRC-assessed TMTV derived from baseline positron emission tomography images using a semi-automatic method with a threshold for TMTV of 2x the SUV mean of the liver.
Results: As of May 1, 2023, 155 patients were enrolled; 154 patients had received ≥1 dose of study treatment. Baseline characteristics were as previously presented (Dickinson et al. N Engl J Med 2022): median prior therapies received was 3 (range: 2-7); 33% of patients had received prior CAR T-cell therapy, and 85% were refractory to their most recent regimen. Median time on study was 25.8 months (range: 0-35).
The IRC-assessed overall response and CR rates were 52% and 40%, respectively. The majority of CRs (40/62; 65%) were ongoing at data cut-off. Median duration of CR (DoCR) was 26.9 months (95% confidence interval [CI]: 18.4-not evaluable [NE]); an estimated 67% of patients with a CR at any time remained in remission at 18 months. PFS and overall survival rates at 12 months in patients with a CR at end of treatment (EOT) were 80% and 90%, respectively. In patients with prior CAR T-cell therapy (n=52), CR rates were consistent with the overall population (N=155; 37% vs 40%, respectively) and median DoCR was 22.0 months (95% CI: 6.7-NE). The safety profile of glofitamab was consistent with what has been described previously, with no new safety signals observed.
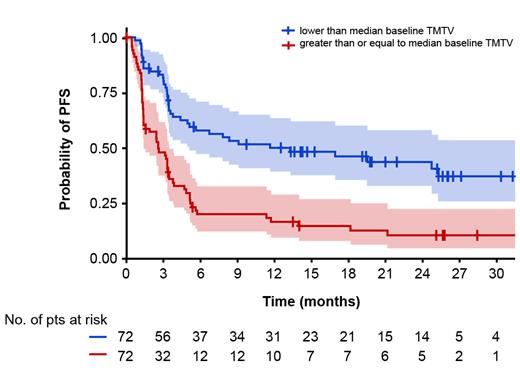
Baseline TMTV was available for 144 patients and the median was 128.7mL (range: 0-3820; data cut-off: January 16, 2023). Higher TMTV was associated with an increased risk of experiencing a Grade ≥2 CRS event. The proportion of patients with Grade ≥2 CRS events in the first, second, third, and fourth TMTV quartiles was 2.8%, 11.1%, 16.7%, and 38.9%, respectively (Chi-square=16.273; degrees of freedom=1; p<0.0001). Patients with a baseline TMTV above or equal to the median (n=72) had a 12-month PFS rate of 16.8% (95% CI: 9.7-29.1), compared with 50.1% (95% CI: 39.5- 63.7) amongst patients with a baseline TMTV below the median (n=72; hazard ratio: 2.6, 95% CI: 1.7-3.9; Figure).
Conclusions: Glofitamab continued to demonstrate durable responses in patients with R/R LBCL, with most patients with CR at EOT still in remission, and no new safety signals observed. These data indicate that there may be potential for favorable long-term outcomes with fixed-duration glofitamab for R/R LBCL. CR rates in patients with prior CAR T-cell therapy were durable and consistent with the overall population. Data from the subgroup analyses showed that higher baseline TMTV was associated with an increased risk of Grade ≥2 CRS and suggested that baseline TMTV may be prognostic for PFS. Updated analyses, with a follow-up of approximately 24 months post EOT, and further TMTV exploratory analyses will be presented.
Disclosures
Hutchings:Martin Hutchings has a Consulting or Advisory Role at Takeda, Roche and Genmab and has received Research Funding from Celgene, Genmab, Roche, Takeda and Novartis.: Consultancy; AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, F. Hoffmann-La Roche Ltd, Takeda: Consultancy; AbbVie, AstraZeneca, Bristol Myers-Squibb, Celgene, Genentech, Genmab, Incyte, Janssen, Merck, Novartis, F. Hoffmann-La Roche Ltd, Takeda: Research Funding; AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, F. Hoffmann-La Roche Ltd, Takeda: Honoraria; AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, F. Hoffmann-La Roche Ltd, Takeda: Membership on an entity's Board of Directors or advisory committees. Carlo-Stella:Janssen Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria; Takeda: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; SOBI: Honoraria, Membership on an entity's Board of Directors or advisory committees. Morschhauser:F. Hoffmann-La Roche Ltd, AbbVie, BMS, Genmab, Gilead, Novartis: Consultancy; F. Hoffmann-La Roche Ltd, Gilead, AbbVie: Membership on an entity's Board of Directors or advisory committees. Falchi:Genentech: Consultancy, Other: Advisory Board, Research Funding; ADC Therapeutics: Other: Advisory Board; Abbvie: Consultancy, Other: Advisory Board, Research Funding; AstraZeneca: Consultancy; Seagen: Other: Advisory Board; Genmab: Consultancy, Research Funding; Roche: Consultancy, Research Funding. Bachy:Takeda: Honoraria; Pfizer: Honoraria, Other: Personal Fees; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Incyte: Honoraria; Novartis: Honoraria, Other: Personal Fees; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Amgen: Research Funding; Roche: Consultancy, Honoraria; Kite, a Gilead Company: Honoraria, Other: Personal Fees. Cartron:Ownards Therapeutics: Consultancy; MabQi: Consultancy; MedxCell: Consultancy; Novartis: Honoraria; Gilead: Honoraria; Emercell: Consultancy; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Janssen: Honoraria; Jansen, Gilead, Novartis, F. Hoffmann-La Roche Ltd, BMS, Abbvie: Honoraria; MedxCell, Ownards Therapeutics, MabQi, Emercell, F. Hoffmann-La Roche Ltd, BMS, Abbvie: Consultancy; MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria. Khan:Allegheny Health Network: Current Employment; Astrazeneca: Consultancy; Kite Pharma, ADC Therapeutics, Genentech, Inc., Astrazeneca, Abbvie, Janssen, BMS, Karyopharm, Pharmacyclics, Seagen: Speakers Bureau. Tani:Abbvie, Jansen-Cilag, Incyte: Membership on an entity's Board of Directors or advisory committees. Bartlett:ADC Therapeutics, Foresight Diagnostics, Kite, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics, Autolus, BMS/Celgene, Forty Seven, Gilead/Kite Pharma, Janssen, Merck, Millennium, Pharmacyclics, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics: Research Funding; Washington University School of Medicine: Current Employment. Salar:Incyte, Beigene, F. Hoffmann-La Roche Ltd: Speakers Bureau; Ipsen: Membership on an entity's Board of Directors or advisory committees. Brody:Kite Pharma: Research Funding; Merck: Research Funding; Kite-Gilead: Research Funding; SeaGen, Merck, BMS, Pharmacyclics, ADC Therapeutics, Epizyme, Genentech, Inc., Kite: Consultancy; SeaGen, Merck, BMS, Pharmacyclics, ADC Therapeutics, Epizyme, Genentech, Inc., Kite: Research Funding; Genentech: Research Funding. Leppä:Bayer, Celgene, Genmab, Hutchmed, Novartis, Nordic Nanovector, F. Hoffmann-La Roche Ltd, (Research Funding to the Institute): Research Funding; Abbvie, Beigene, Gilead, Incyte, Novartis, Orion, Pfizer, F. Hoffmann-La Roche Ltd, Sobi: Consultancy; Gilead, F. Hoffmann-La Roche Ltd, Novartis, Incyte: Honoraria. Baumlin:F. Hoffmann La Roche Ltd: Current Employment. Mulvihill:F. Hoffmann La Roche Ltd: Current Employment, Current equity holder in private company, Divested equity in a private or publicly-traded company in the past 24 months. Relf:F. Hoffmann La Roche Ltd.: Current equity holder in publicly-traded company; F-Star Therapeutics: Divested equity in a private or publicly-traded company in the past 24 months; Roche Products Ltd: Current Employment. Xie:F. Hoffmann La Roche Ltd (Canada): Current Employment. Kaufman:Genentech, Inc. / F. Hoffmann-La Roche Ltd: Current Employment; F. Hoffmann La Roche Ltd: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Lundberg:F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; F. Hoffmann-La Roche Ltd: Current Employment; F. Hoffmann La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Dickinson:F. Hoffmann-La Roche Ltd: Other: travel, accommodation, expenses; F. Hoffmann-La Roche Ltd, Amgen, MSD, Janssen, Bristol-Myers Squibb, Novartis, Gilead Sciences, Abbvie: Honoraria; Novartis, F. Hoffmann-La Roche Ltd, Takeda, Celgene, MSD, Abbvie, Lilly: Research Funding; Novartis, F. Hoffmann-La Roche Ltd, Bristol-Myers Squibb, Gilead Sciences, Janssen, Abbvie, Genmab: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal